Nitrate
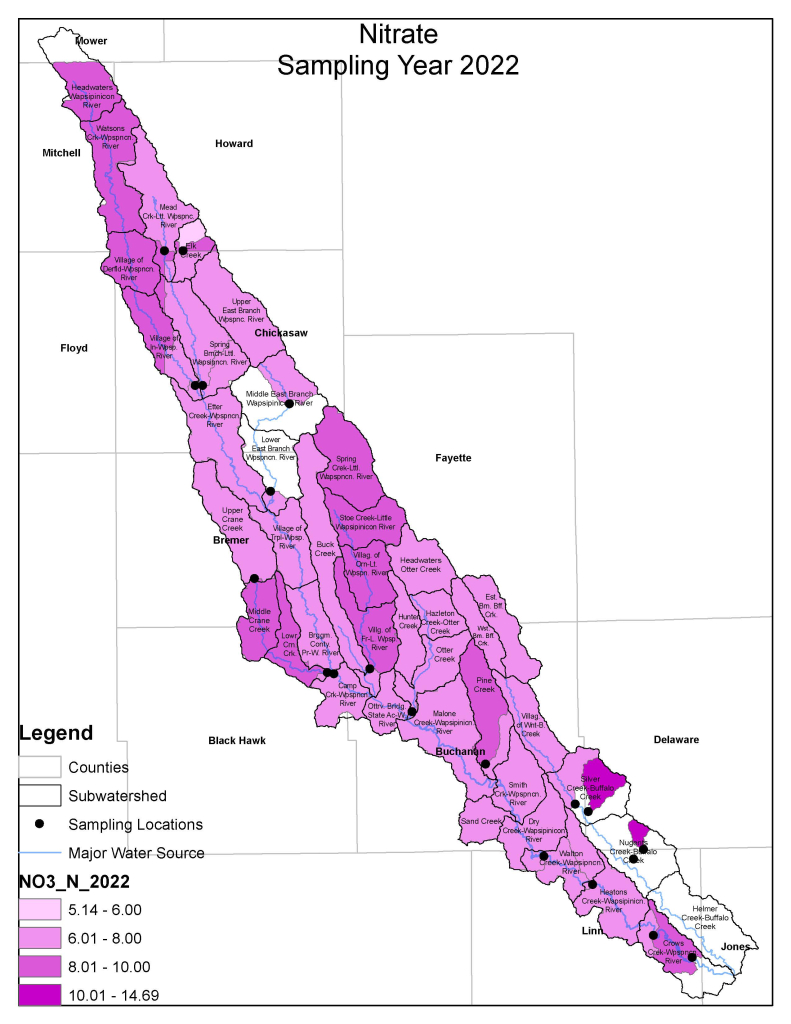
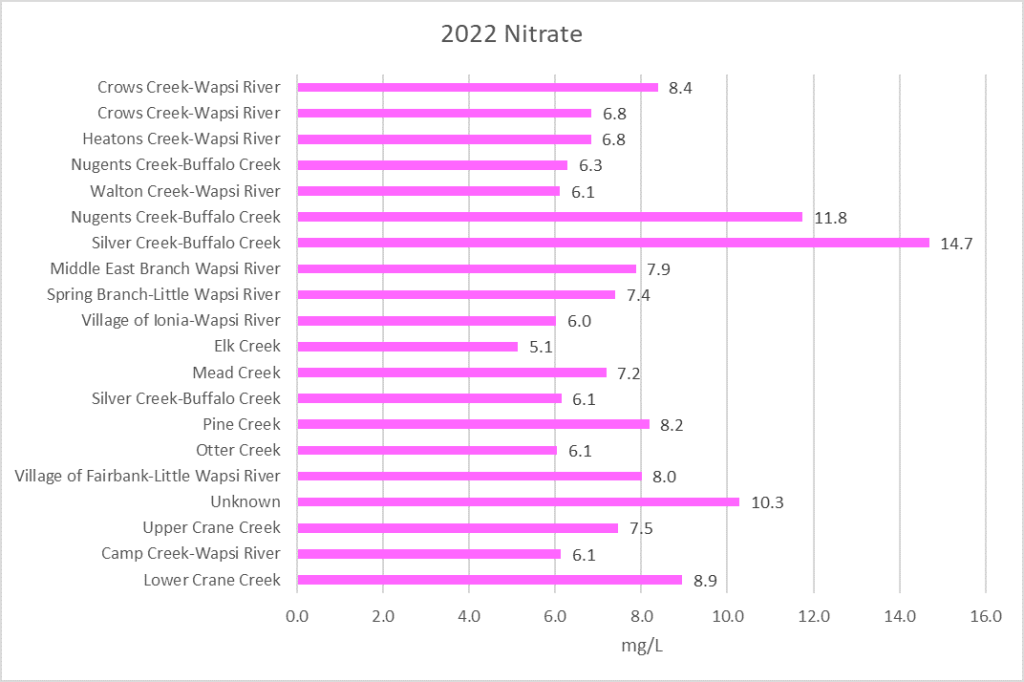
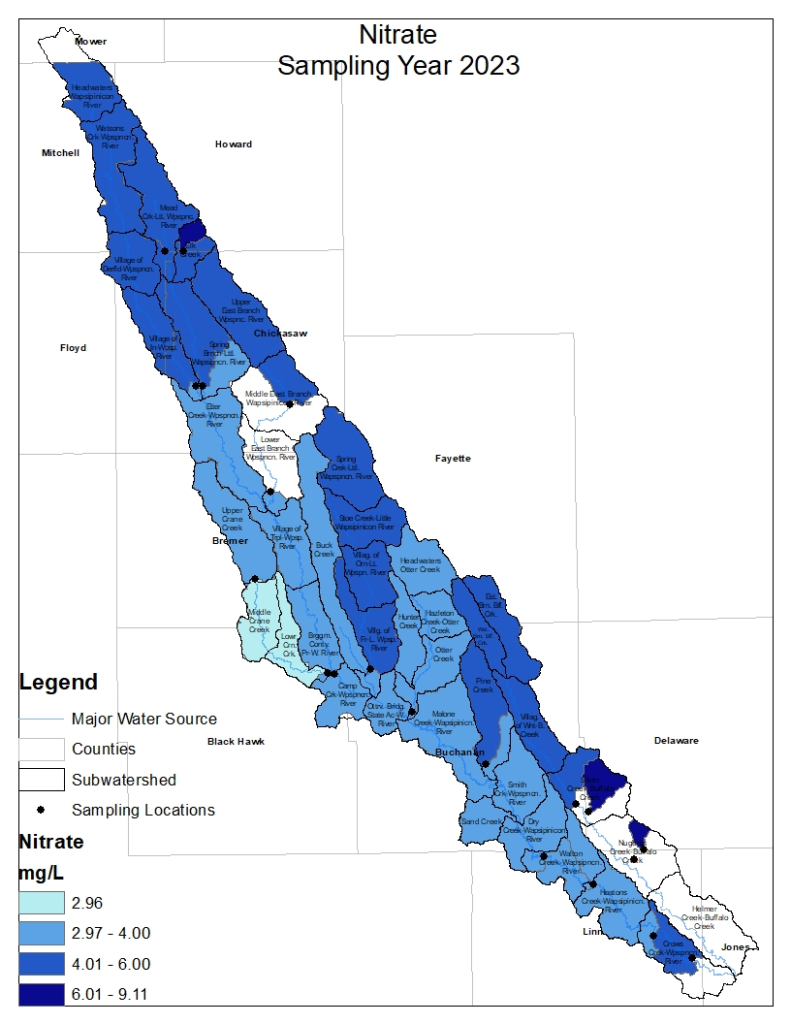
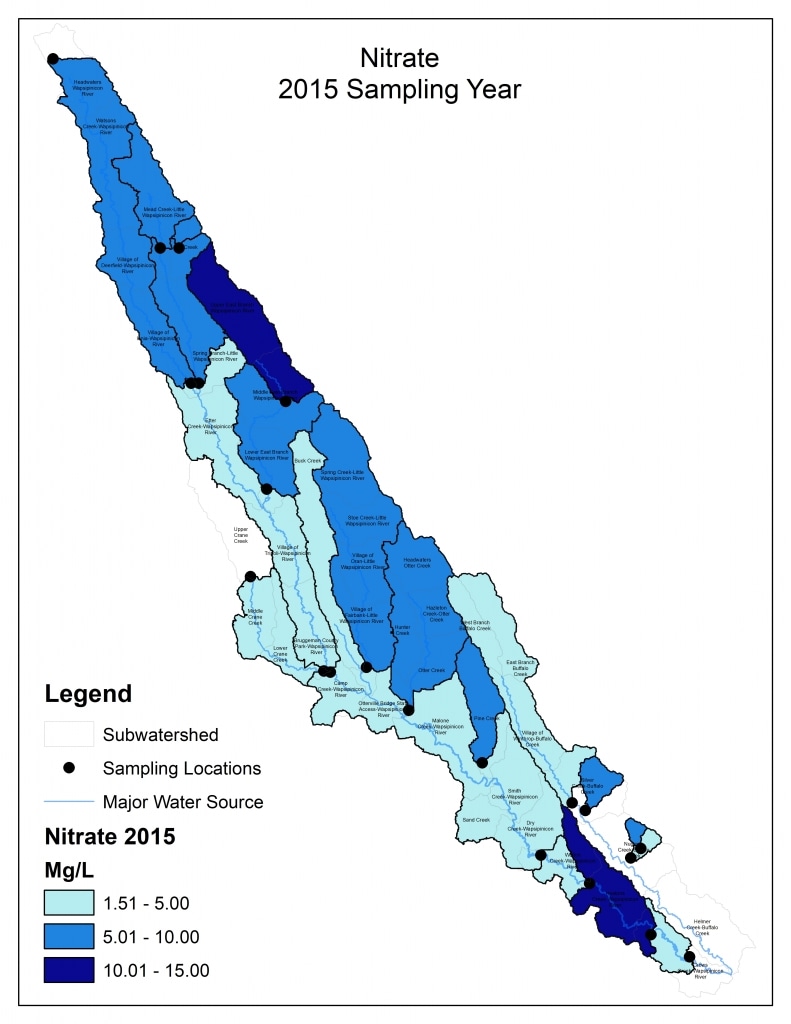
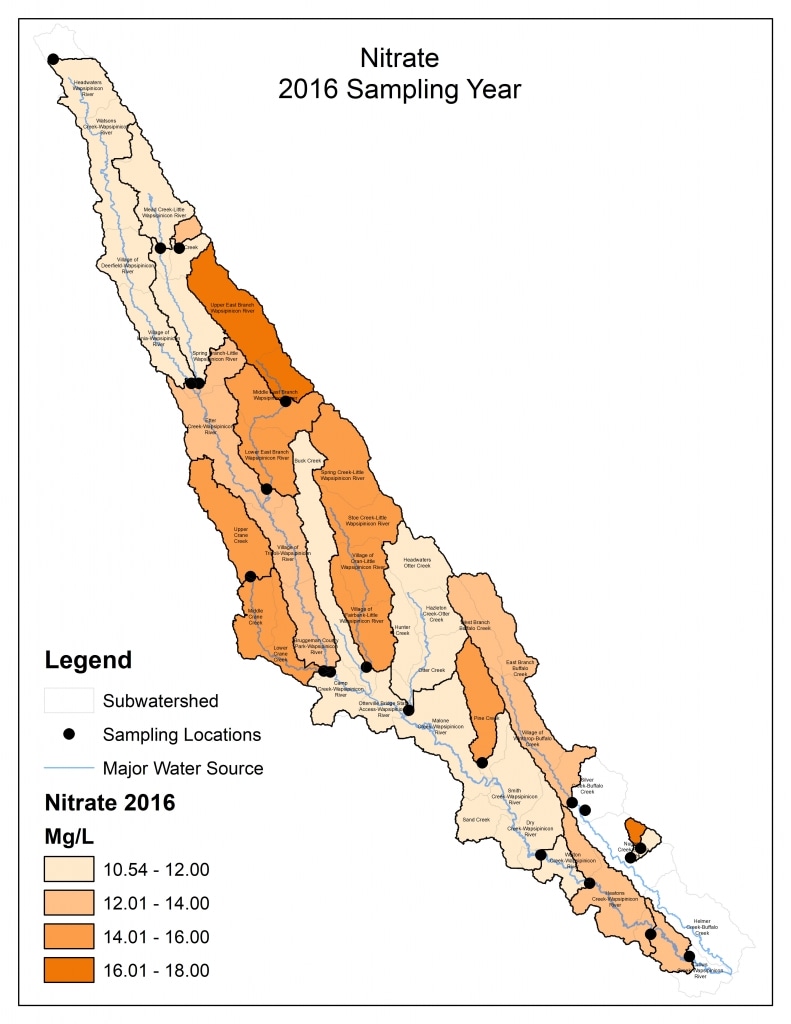
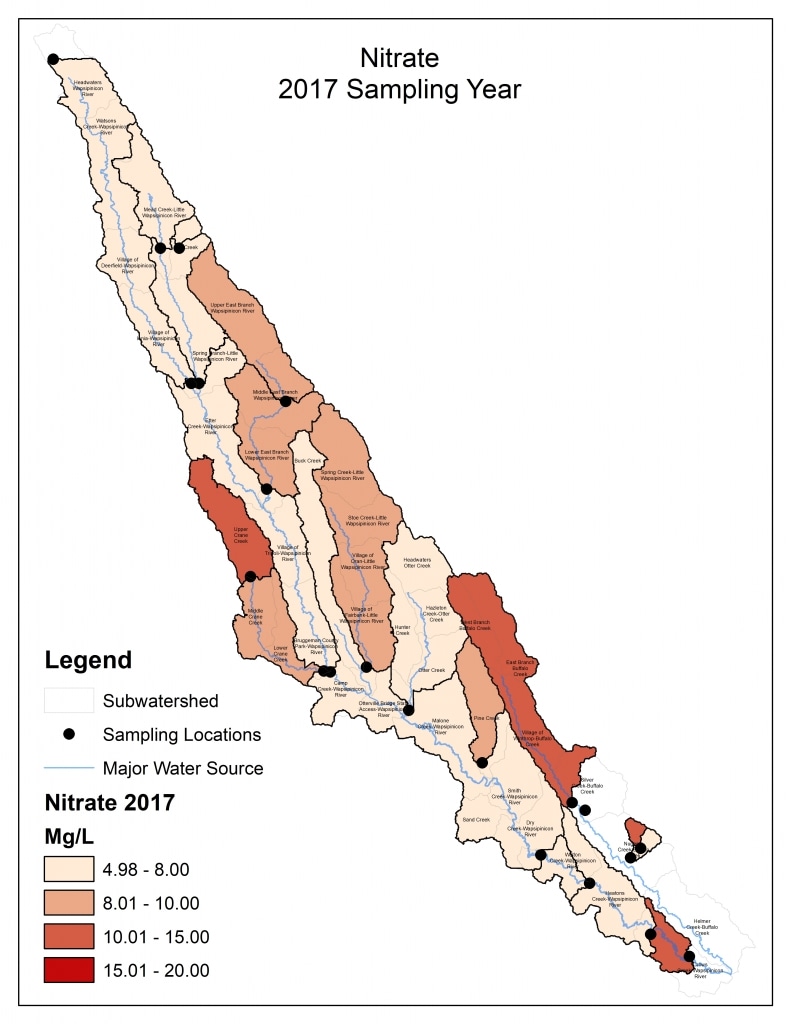
Nitrogen is an essential plant nutrient, but excess nitrogen can cause water quality problems. Too much nitrogen and phosphorus in surface waters causes nutrient enrichment, increasing aquatic plant growth and changing the types of plants and animals that live in a waterbody. This process, called eutrophication, can also affect other water quality parameters such as pH and dissolved oxygen. Nitrate and nitrite are two forms of nitrogen. Nitrate is very easily dissolved in water and is more common in streams. Sources of nitrate include soil organic matter, animal waste, decomposing plants, sewage, and fertilizers. Because nitrate is very soluble in water it can move readily into streams. Nitrite is another form of nitrogen that is less common because it is quickly converted to nitrate or returned back to the atmosphere as nitrogen gas. Due to its instability, detectable levels of nitrite in streams and lakes are uncommon. Detectable nitrite levels in streams and lakes may indicate a relatively fresh source of ammonia. The amount of nitrate or nitrite dissolved in water is reported as Nitrate-N (nitrate expressed as the element nitrogen) or Nitrite-N in milligrams per liter of water (mg/L). According to the EPA, Iowa’s nitrate water quality standard for fish and water consumption is 10 mg/L as Nitrate-N. The concentration of Nitrate-N in water may vary greatly depending on season and rainfall, fertilizer application rates, tillage methods, land use practices, soil types, and drainage systems. Consistently high nitrate readings (over 10 mg/L) is a great concern and further investigation should be conducted.
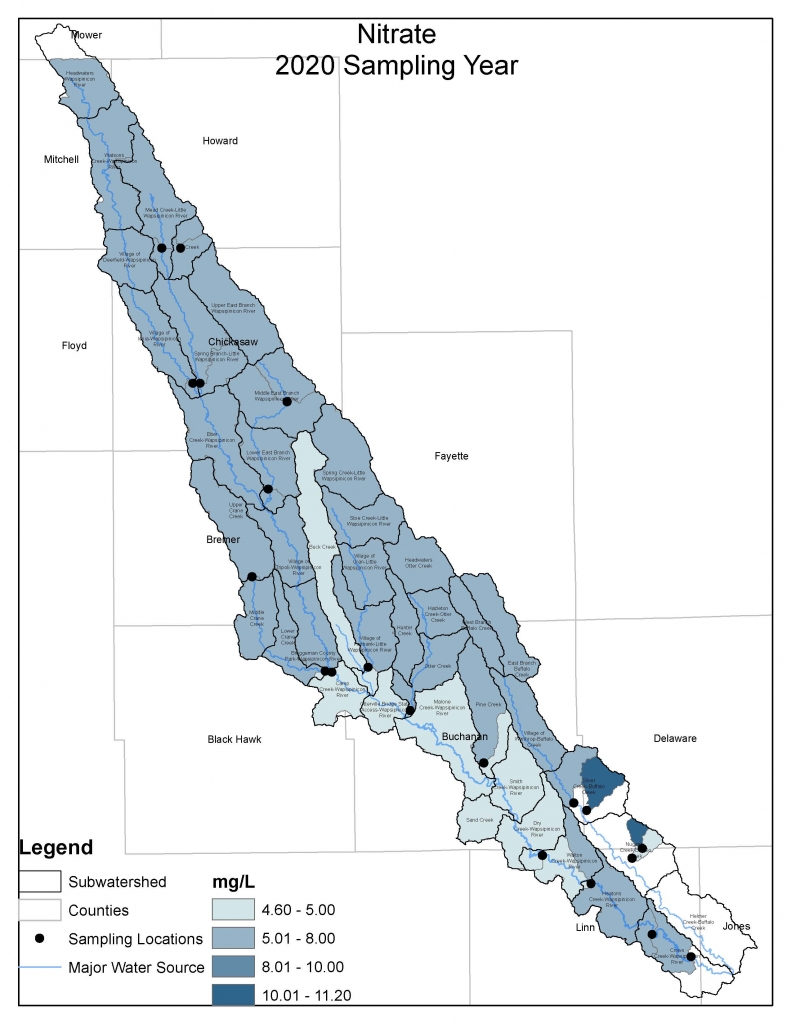
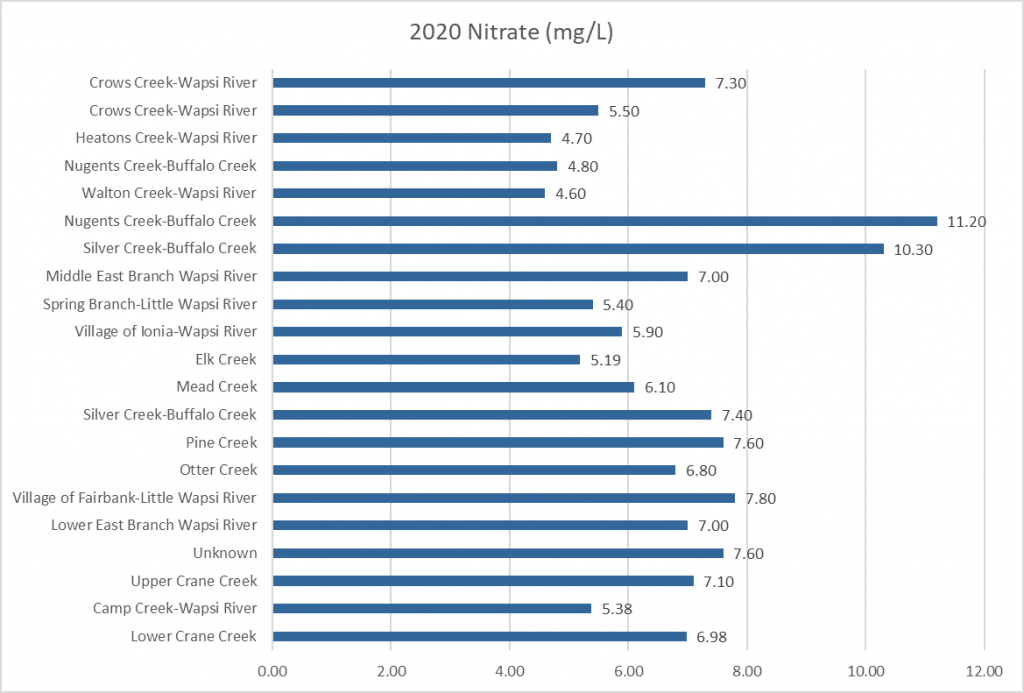
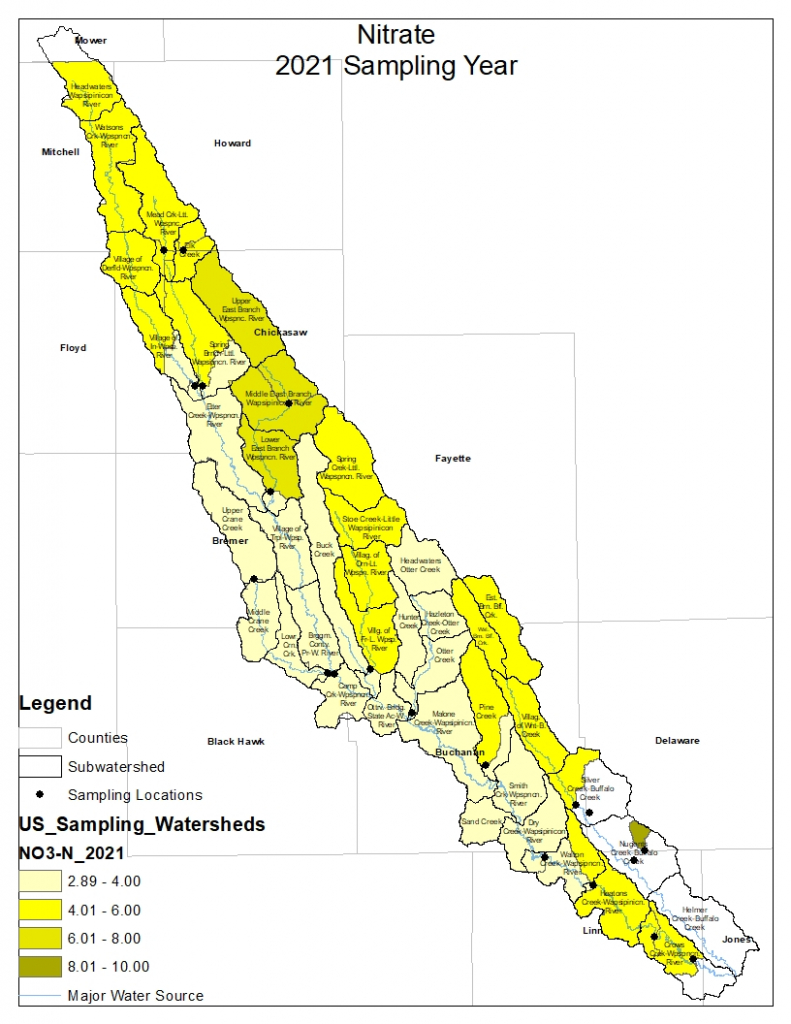
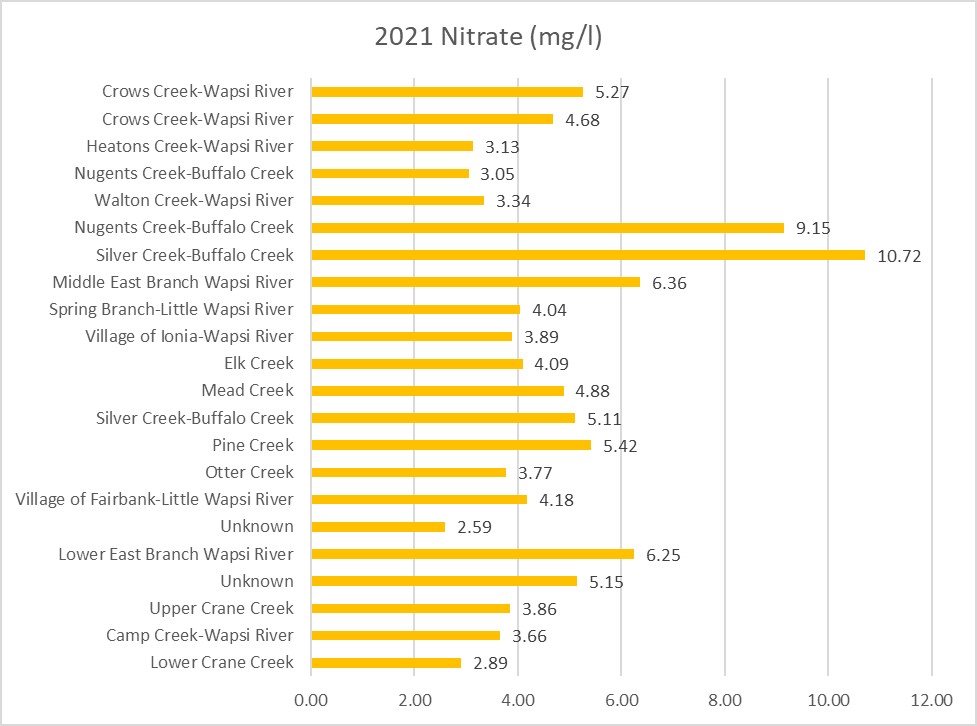
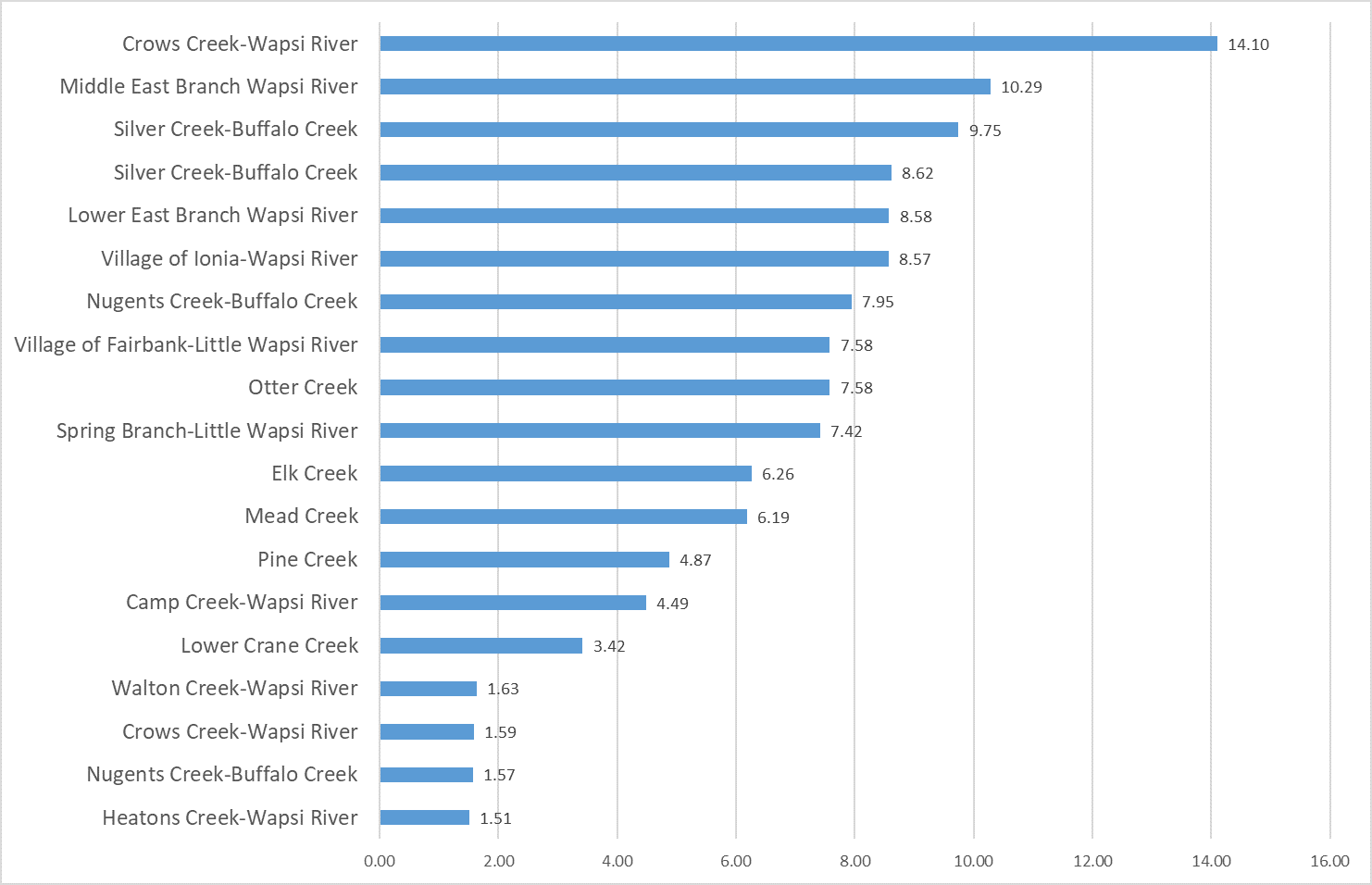
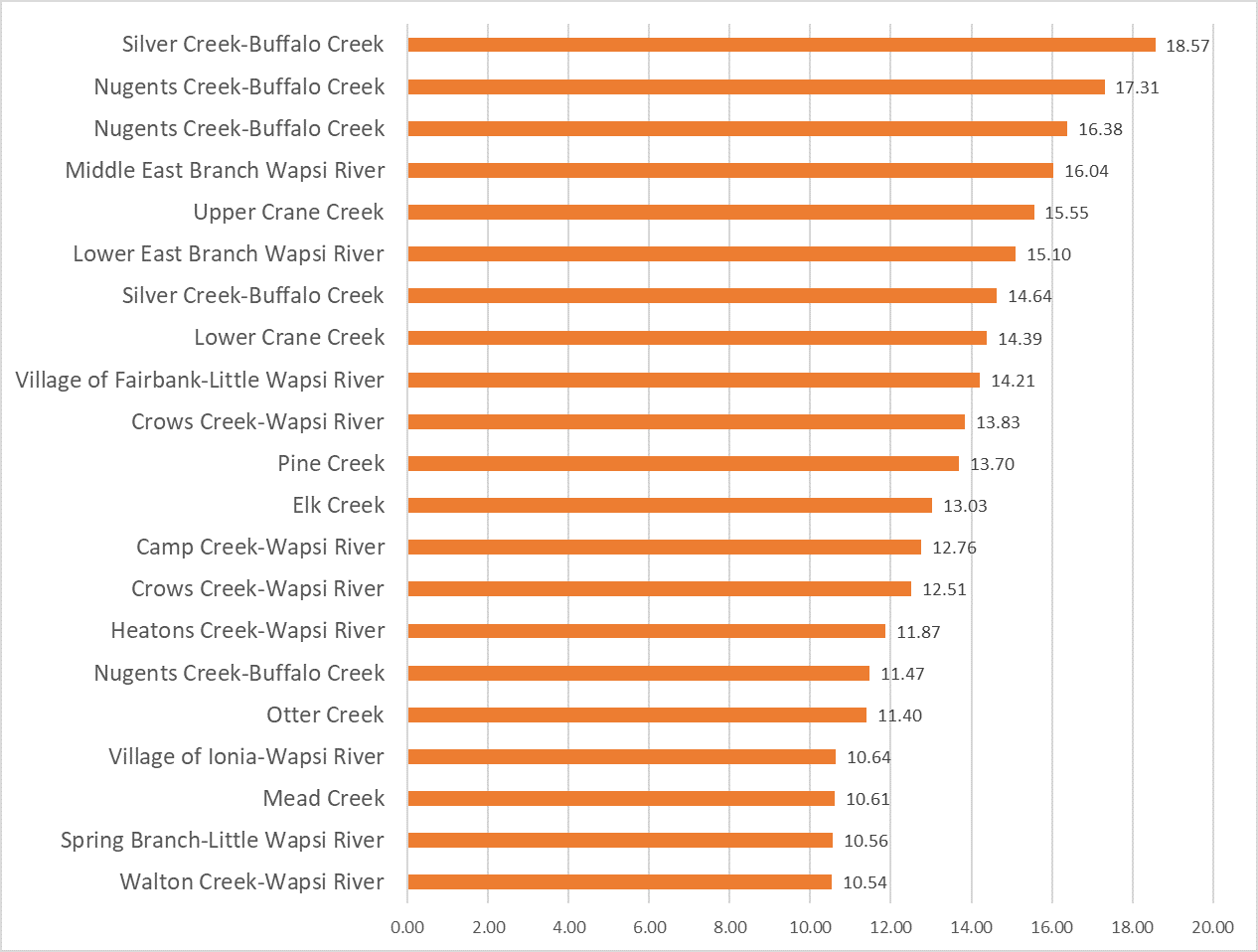
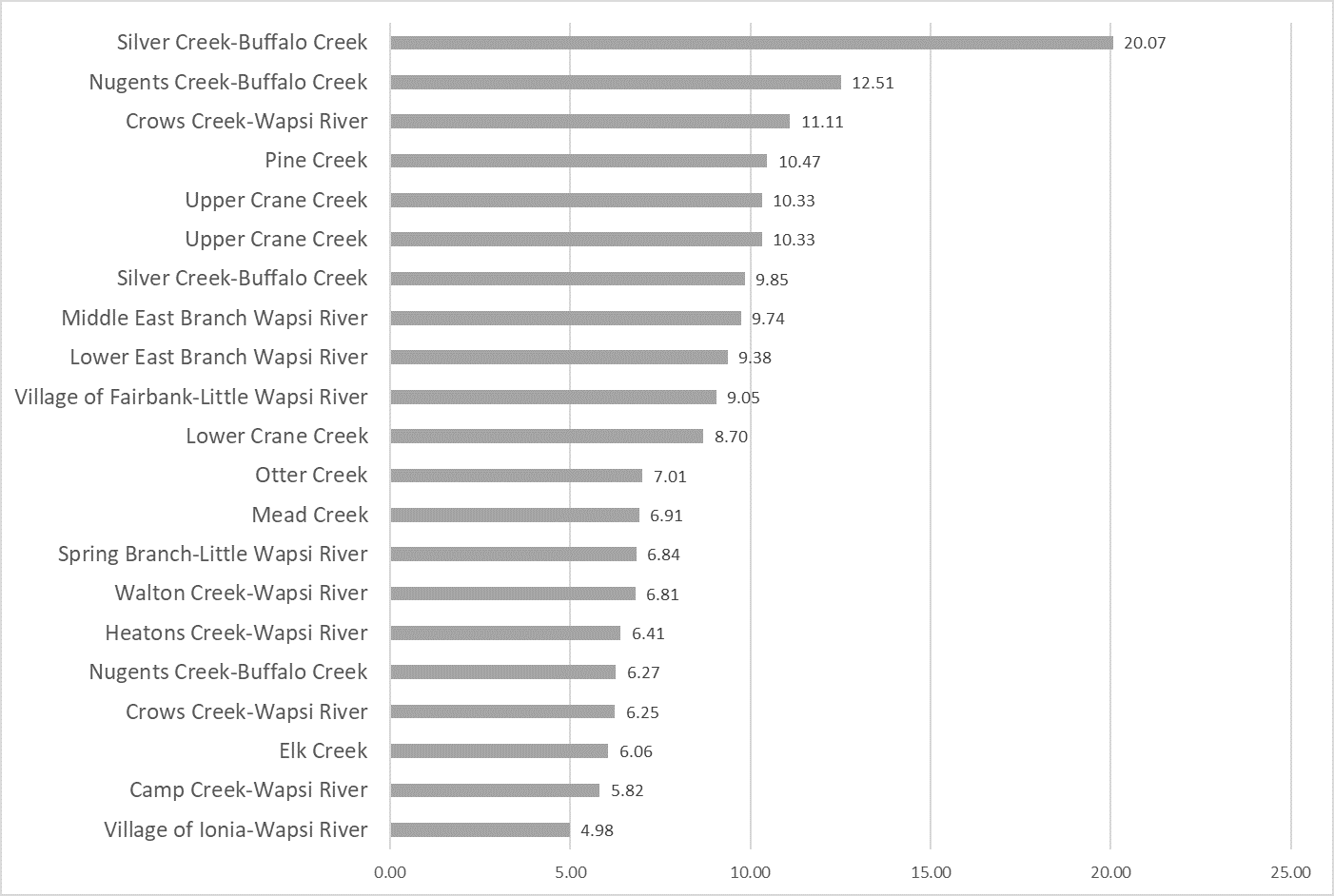
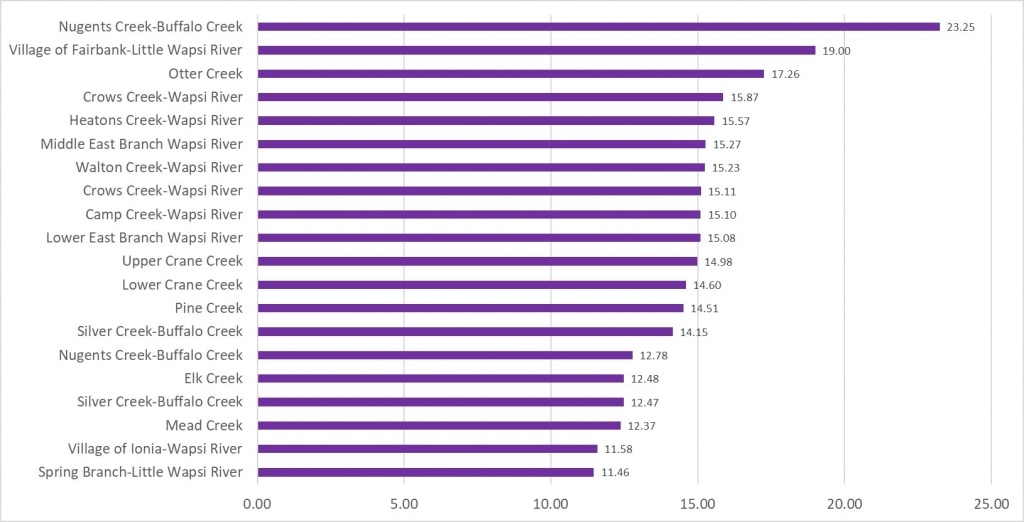
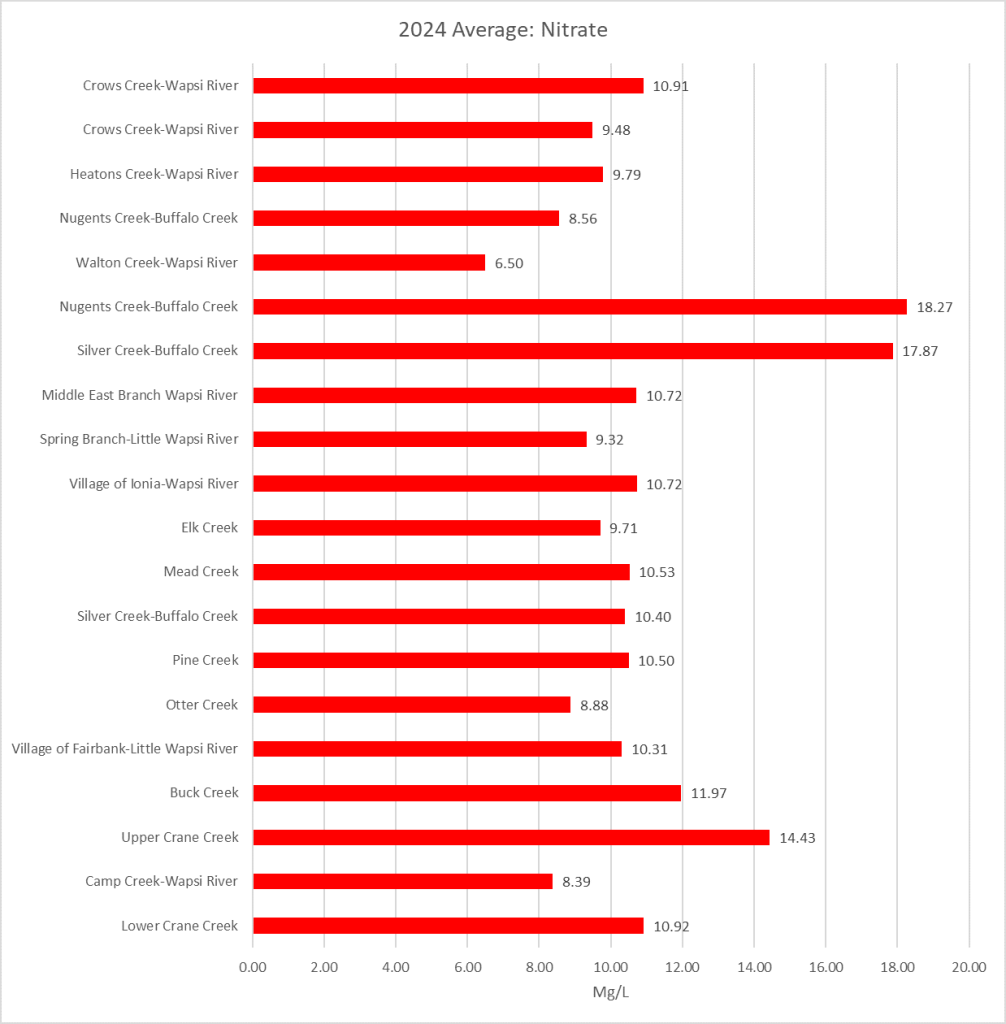
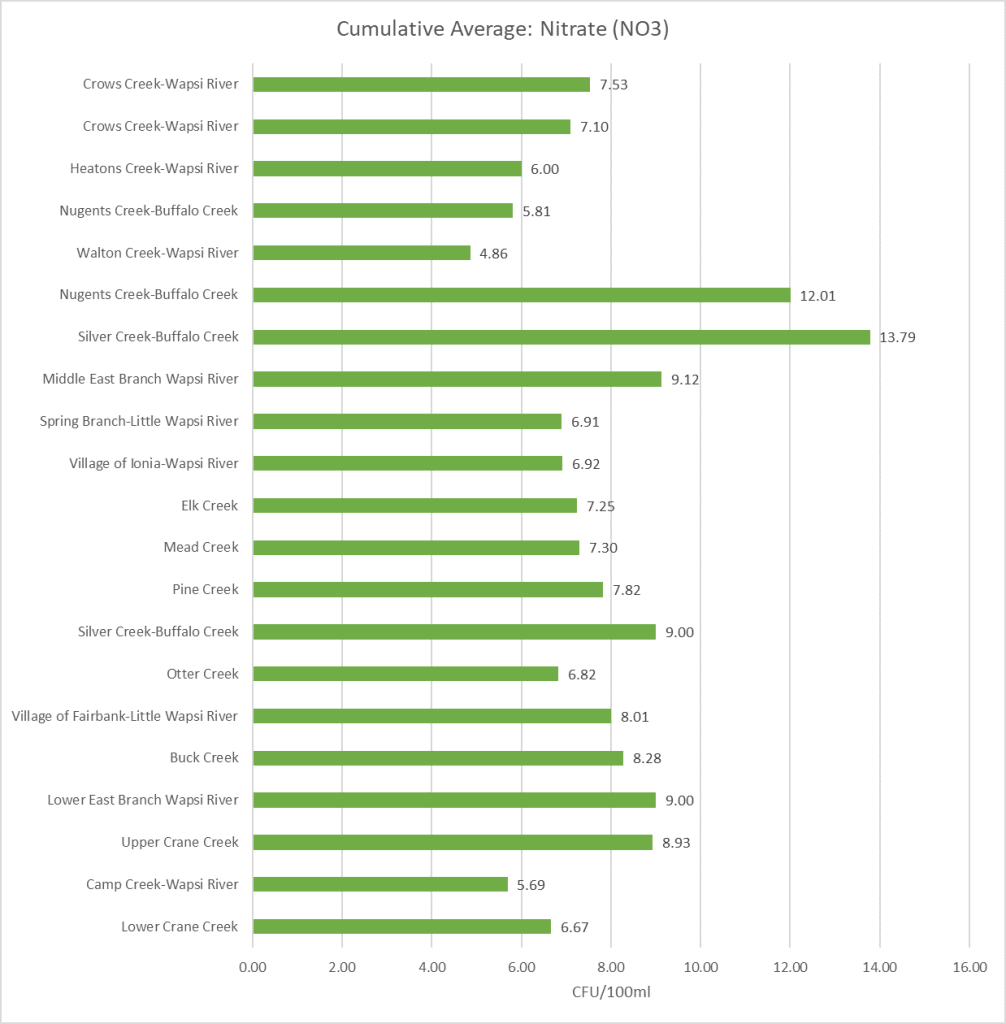
Results by Sampling Year
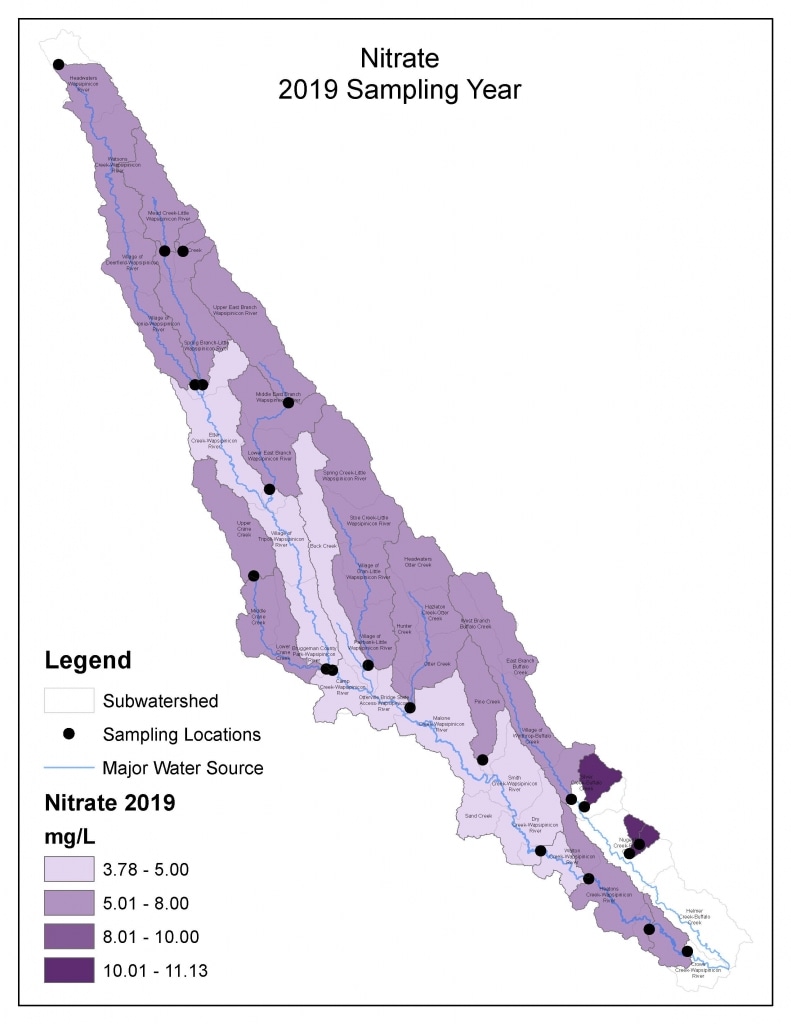 2019 Nitrate
2019 Nitrate
(Mg/l)